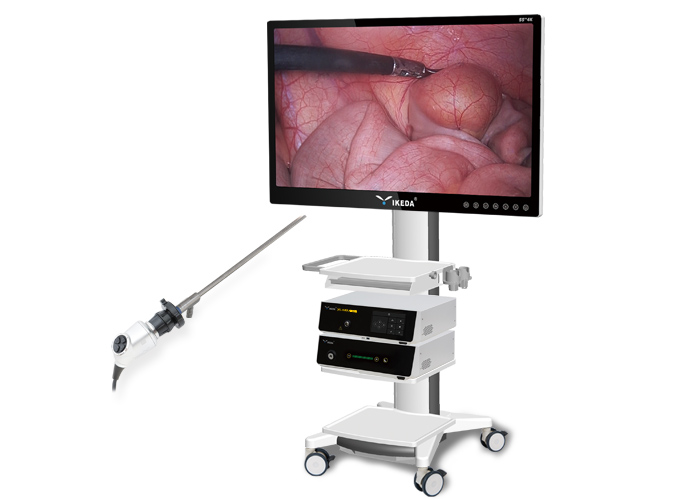
YIKEDA announced that the company's 4K medical endoscope camera system has been approved by the Jiangsu Provincial Drug Administration and recently obtained the medical device registration certificate of the People's Republic of China.
Registration certificate number: Su Xie Zhu Zhun 20222060603.
Certificate approval date: January 24, 2022, valid until: January 23, 2027.