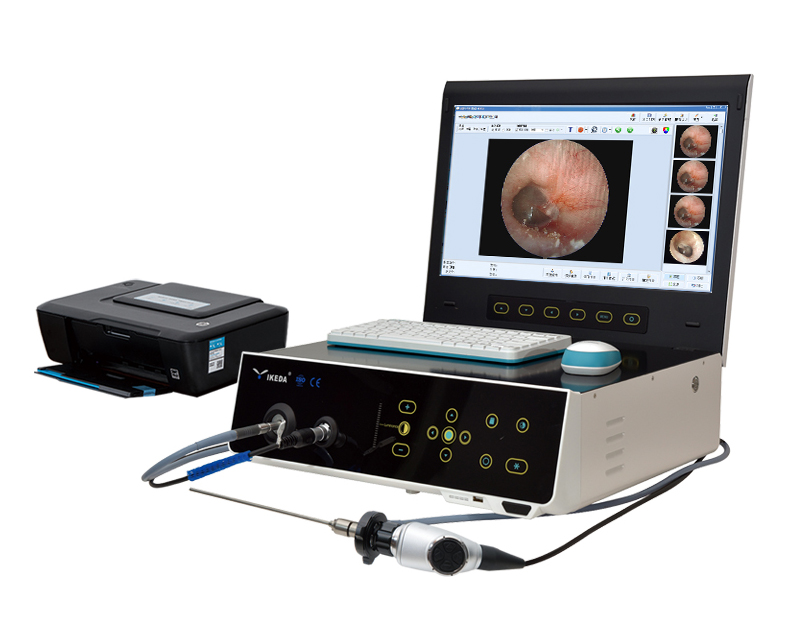
YIKEDA issued an announcement that the company’s All-in-one Endoscope Imaging System has been approved by the Jiangsu Provincial Drug Administration, and recently obtained the People’s Republic of China Medical Device Registration Certificate, the registration number: Su Xie Zhuzhun 20212061298, the certificate approval date: 2021 September 6, 2026, valid until: September 5, 2026; scope of application: it is connected to an endoscope in clinical use, and is used to convert the visual image of the endoscope into a video image, and provide the endoscope at the same time light source. Model and specifications: YKD-9100, YKD-9101, YKD-9102, YKD-9103, YKD-9105, YKD-9106, YKD-9107;
The All-in-one Endoscope Imaging System consists of a control host, cold light source, camera, display screen, optical interface, imaging workstation and printer (optional).